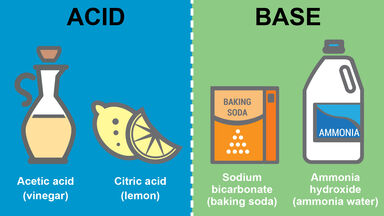
Citric acid is also distinguished from tartaric acid by the fact that an ammonia solution of silver tartrate produces a brilliant silver mirror when boiled, whereas silver citrate is reduced only after prolonged ebullition.
Kekule (Ann., 1883, 221, p. 230), however, reinvestigated this acid; he showed that it was dibasic and not tribasic; that it gave tartaric acid on reduction; and, finally, that it was dioxytartaric acid, HOOC C(OH) 2 C(OH) 2 COOH.
The character of the acidity, however, changes, the free tartaric acid gradually disappearing, forming bitartrate of potash and being otherwise broken up. On the other hand, the free malic acid increases and the tannin decreases.
It has been found, for instance, that in the case of the mannitic disease the action of the micro-organism may be checked, or prevented altogether, by bringing the acidity of the must up to a certain level by the addition of a small quantity of tartaric acid.
In repeating and extending the experiments of Haiiy much later, Sir David Brewster discovered that various artificial salts were pyro-electric, and he mentions the tartrates of potash and soda and tartaric acid as exhibiting this property in a very strong degree.