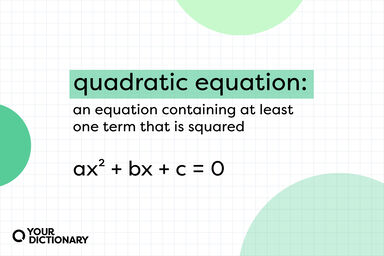Nernst Equation Definition
noun
An electrochemical equation that relates the reduction potential of a half-cell (or the total voltage, i.e. the electromotive force, of the full cell) at any point in time to the standard electrode potential, temperature, activity, and reaction quotient of the underlying reactions and species used.
Wiktionary
Other Word Forms of Nernst Equation
Noun
Singular:
Nernst equationPlural:
Nernst equationsOrigin of Nernst Equation
Named after the German physical chemist Walther Nernst , who formulated it.
From Wiktionary
Find Similar Words
Find similar words to Nernst equation using the buttons below.