Geisel (Ber., 1904, 37, p. 1 573; 1905, 38, p. 2659), who also obtained it by dissolving sulphur in liquid ammonia.
It forms addition compounds similar to those formed by stannic chloride, and combines with ammonia to form TiCl 4.8NH 3 and TiC1 4.6NH 3, both of which with liquid ammonia give titanamide, Ti(NH2)4.
The iodide combines with liquid ammonia to form ZrI 4.8NH 3 i and with ether to give Zr14.4(C2H5)20.
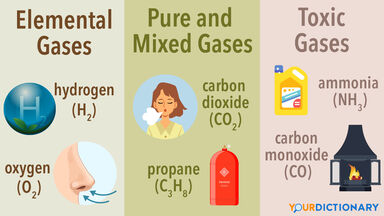
Liquid ammonia possesses strong ionizing powers, and solutions of salts in liquid ammonia have been much studied.
Sodium trioxide, Na 2 O 31 is said to be formed from an excess of oxygen and a solution of sodammonium in liquid ammonia.