A similar model was proposed with hydroxyl ions hydrogen bonding to surface H causing polarization and conductivity.
In this case it is the radius of the hydrogen-bonding hydrogen-bonding hydrogens which is reduced, rather than the radius of the central nitrogen itself.
Stronger hydrogen bonding causes the O···O distance to be shorter, so easing the further shortening required for transfer.
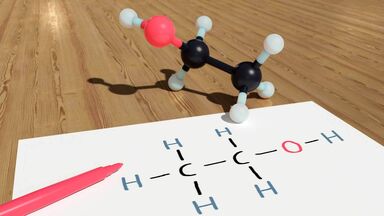
Hydrogen fluoride is very soluble due to hydrogen bonding with water molecules.
Such hydrogen bonding induces a more negative charge on the carboxyl oxygen atoms leading to an increase in the carboxyl oxygen atoms leading to an increase in the carboxylate pK a.