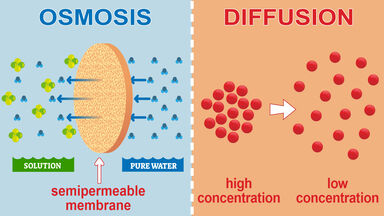
His early work on the movements of gases led him to examine the spontaneous movements of liquids, and as a result of the experiments he divided bodies into two classes - crystalloids, such as common salt, and colloids, of which gum-arabic is a type - the former having high and the latter low diffusibility.
He also proved that the process of liquid diffusion causes partial decomposition of certain chemical compounds, the potassium sulphate, for instance, being separated from the aluminium sulphate in alum by the higher diffusibility of the former salt.
Graham (Chemical and Physical Researches) recommended dialysis as the best mode of preparing gummic acid, and stated that the power of gum to penetrate the parchment septum is 400 times less than that of sodium chloride, and, further, that by mixing the gum with substances of the crystalloid class the diffusibility is lowered, and may be even reduced to nothing.