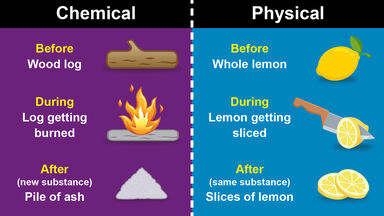
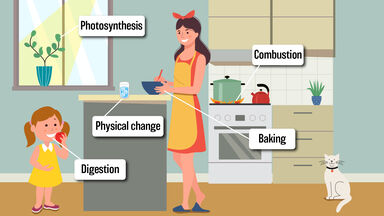
Some people find their hair is resistant to perms, and a stronger solution may need to be applied to boost the chemical change.
It is evident that if our experiments are solely directed to the verification of this law, they should, if possible; be carried out in a hermetically closed vessel, the vessel and its contents being weighed before and after the chemical change.
But on account of experimental errors in weighing and measuring, and through loss of material in the transfer of substances from one vessel to another, such analyses are rarely trustworthy to more than one part in about Soo; so that small changes in weight consequent on the chemical change could not with certainty be proved or disproved.
The distribution of weight in chemical change is readily expressed in the form of equations by the aid of these symbols; the equation 2HC1+Zn =ZnCl2+H2, for example, is to be read as meaning that from 73 parts of hydrochloric acid and 65 parts of zinc, 136 parts of zinc chloride and 2 parts of hydrogen are produced.
Similar relations were found to hold and the amounts of chemical change to be the same for the same electric transfer as in the case of solutions.