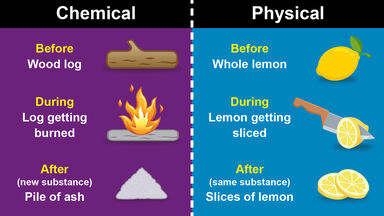
As an example, we may apply this condition to the case of change of state.
In some cases, however, they are filled with fused acetate of soda; this salt is solid when cold, but when the can containing it is heated by immersion in hot water it liquefies, and in the process absorbs heat which is given out again on the change of state back to solid.
The methods depending on change of state are theoretically the simplest, since they do not necessarily involve any reference to thermometry, and the corrections for external loss of heat and for the thermal capacity of the containing vessels can be completely eliminated.
The most instructive example of the application of relations (I) and (2) is afforded by the change of state of a substance at constant temperature and pressure.
The simplest application of the thermodynamic potential is to questions of change of state.