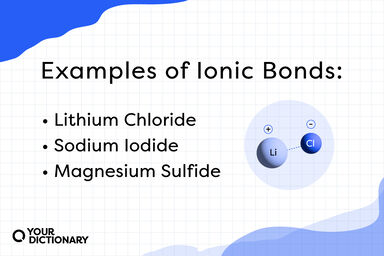
Doubtless, the excess of any soluble mineral salt or salts interferes with the osmotic absorption of the roots; and although calcium carbonate is insoluble in pure water, it is slightly soluble in water containing carbon dioxide.
There are, therefore, a number of agencies, all of which operate in shoal waters on the lee side of islands, or in shallow lagoons in such regions as the Bahamas, and the result of all these is to throw down calcium carbonate from solution in sea-water as minute needle-shaped crystals or little balls of aragonite.
Obviously no more than this is possible until physiologists are able to state much more precisely than at present what is the influence of common salt on the plants of salt-marshes, of the action of calcium carbonate on plants of calcareous soils, and of the action of humous compounds on plants of fens and peat moors.
However, until more is known of the exact chemical composition of naturalas contrasted with agriculturalsoils, and until more is known of the physiological effects of lime, it is impossible to decide the vexed question of the relation of limeloving and lime-shunning plants to the presence or absence of calcium carbonate in the soil.
The Kassner process for the manufacture of oxygen depends upon the formation of calcium plumbate, Ca2Pb04, by heating a mixture of lime and litharge in a current of air, decomposing this substance into calcium carbonate and lead dioxide by heating in a current of carbon dioxide, and then decomposing these compounds with the evolution of carbon dioxide and oxygen by raising the temperature.