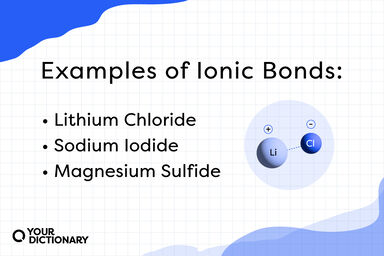
Gay-Lussac in 181q, is usually obtained in the form of its barium salt by suspending freshly precipitated hydrated manganese dioxide in water and passing sulphur dioxide into the mixture until all is dissolved; the barium salt is then precipitated by the careful addition of barium hydroxide.
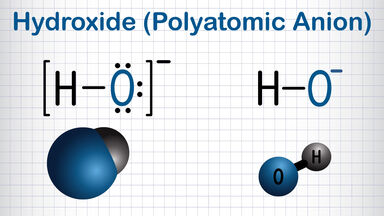
Aqueous alcohol becomes turbid when mixed with benzene, carbon disulphide or paraffin oil; when added to a solution of barium oxide in absolute alcohol, a white precipitate of barium hydroxide is formed.
These alkyl substitution products are important, for they lead to the synthesis of many organic compounds, on account of the fact that they can be hydrolysed in two different ways, barium hydroxide or dilute sodium hydroxide solution giving the socalled ketone hydrolysis, whilst concentrated sodium hydroxide gives the acid hydrolysis.