
Pull on your lab coat. Chemical terms and definitions are on the horizon. Dive into the different terms used in chemical energy, reactions, mixtures, and more.
Common Chemistry Terms
Trying to wade through the murky waters of chemistry terms can get confusing fast. Keep things clear by exploring the common terms in chemistry definitions.
- alkali metals - the metals in the first periodic table column, minus hydrogen
- allotropes - all the different forms elements can exist in
- alloy - metal mixtures with multiple elements
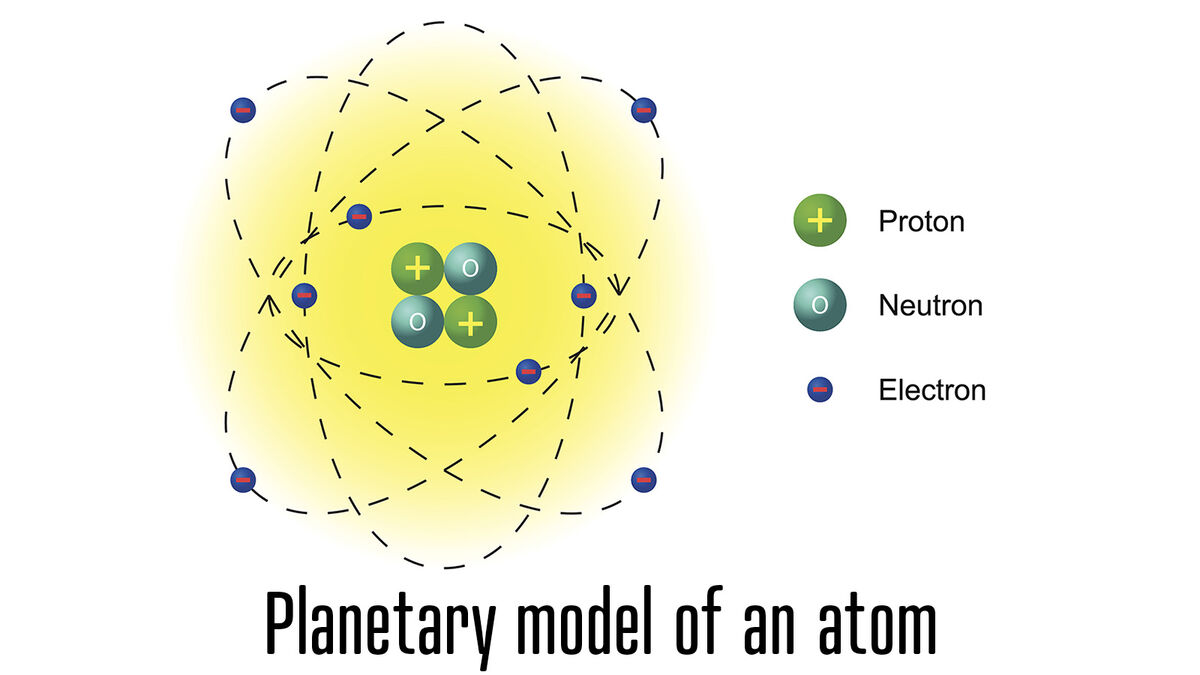
- atom - smallest unit of matter
- biochemistry - exploration of chemical processes in biological organisms
- carbon cycle - movement of carbon in the biosphere
- conductor - metal that electrical energy easily flows through
- density - the measurement of compactness of molecules in a substance
- electron - negatively charged particle that orbits the nucleus of an atom
- element - substance made of one type of atom
- ion - atom with a charge
- isotopes - element with a different number of neutrons than standard
- malleable - flexibility of a substance to be molded or shaped
- metal - malleable and shiny substances good at conducting electricity
- molecule - substance created from two or more atoms bonded together
- neutron - atom particle without charge
- noble gas - stable elements on the periodic table in column 18
- nucleus - the center or core of an atom
- periodic table - elements arranged by atomic number in a table format
- proton - positively charged particle in an atom
- valence electrons - electrons that participate in chemical bonding
Solution and Mixture Chemistry Terms
Get your stirring sticks ready because it is time to look at solutions in chemistry. Explore different chemistry terms related to mixtures and solutions.
- absorption - the process where something soaks up something else
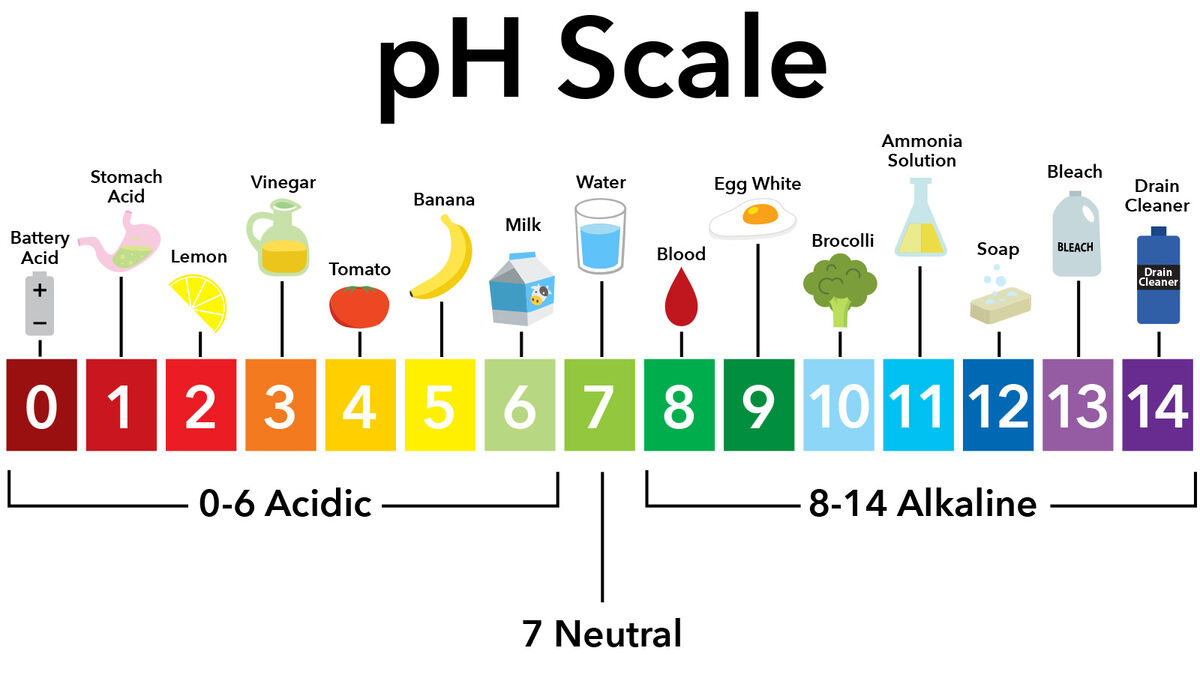
- acid - compound with a pH of less than 7
- scid solution - a solution with larger quantities of hydrogen ions giving it a pH lower than 7
- aqueous solution - solution that uses water as the solvent
- base - substance with pH higher than 7
- bunsen burner - burner used for chemistry experiments
- dilution - process of making a liquid weaker
- distillation - process where liquid is boiled, and gas is recovered and cooled like water distillation
- mass - how much matter is in a substance
- mixture - when elements are physically combined, but not chemically
- pH scale - measurement of how basic or acidic a substance is
- pressure - measurement of force applied to something
- salts - compounds formed by acid and base reactions
- solution - when a substance dissolves in another like sugar in water
- solvent - something that dissolves other substances
Chemical Reaction Terms
Have your goggles at the ready! You are going to see why chemical bonds occur through chemistry terms.
- activation energy - the minimum amount of energy needed for a chemical reaction to occur
- bond - atom attractions that form substances
- catalyst - substance used to make a reaction happen or happen quicker
- chemical reaction - when a substance changes into a new substance (i.e. wood to ash)
- combustion - process of burning
- compound - when two or more elements combine
- covalent bond - bond where electrons are shared
- electrolysis - chemical reaction using electricity
- endothermic reaction - reaction where heat is absorbed
- enzyme - substance that works as a catalyst for reactions
- exothermic reaction - the release of heat in a reaction
- fusion - where two things are joined
- inhibitor - substance that stops or prevents a reaction
- ionic bond - bond where electrons are transferred
- metallic bond - bond between metallic elements
- oxidation - when electrons are lost during a chemical reaction
- potential energy - the potential something has to do work
- product - result of a chemical reaction
- reactant - substance that initiates a chemical reaction
- temperature - measurement of kinetic energy in a substance
The Interesting World of Chemistry
Without chemical reactions, fireworks would be a thing of fiction. Get lost in more science fun by looking at examples of kinetic energy. And if heat is more your thing, try out heat energy examples. It’s getting hot, hot, hot!