Science is all about order. And, to keep things straight, you have tons of different terminology. Sometimes, such as in the case of molecules vs. compounds, it gets confusing. Here’s why.
Compounds and molecules are related because all compounds are molecules. Easy enough. However, the difference between compound and molecule is not all molecules are compounds. Is your head spinning yet? To clear everything up, it’s important to break down molecule vs. compound into their basic definitions and look at their various parts.
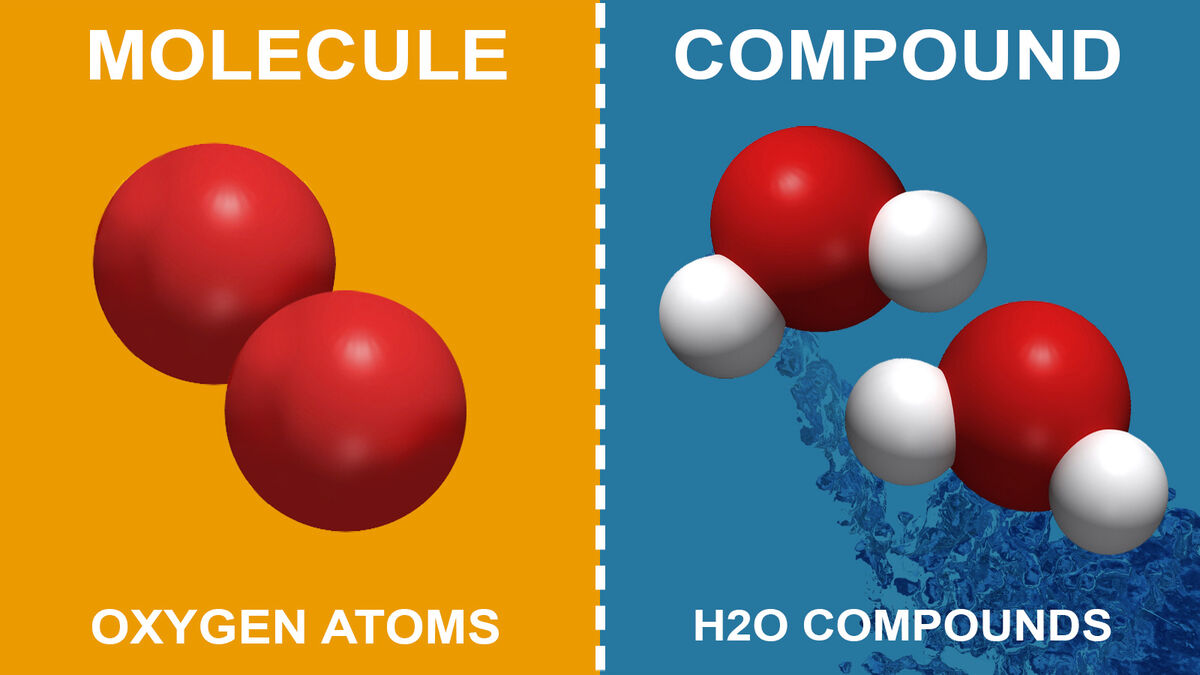
What Is a Molecule?
To understand molecules, it’s important to first clear up atoms. Atoms are these fun structures with electrons, neutrons, and protons in them. Electrons and protons have a negative and positive charge respectively.
Atoms like to be balanced. Therefore, they will chemically bond with other atoms to be balanced. When an atom bonds with other atoms, either the same type (O2) or a different type (H2O), it’s called a molecule. Molecules are tiny so you can’t see them with the naked eye. However, scientists can break them down to their atomic parts. For example, water is made of two hydrogen atoms and one oxygen atom (H2O).
Molecule Examples
There are lots of different molecules. Here are just a few examples:
- oxygen (O2)
- carbon dioxide (CO2)
- sulfuric acid (H2SO4)
- ozone (O3)
- nitrogen (N2)
Notice how all the examples have at least two atoms in them? That’s what makes them a molecule.
What Is a Compound?
Now it’s time to look at compounds. Remember how compounds are molecules? Here is why. A compound is created when atoms of two different elements combine. You still have two atoms combining together, so it's definitely a molecule. But for it to be a compound, those elements must be different. For example:
- Oxygen (O2) is only a molecule because it is two oxygen atoms bonded.
- CO2 is a compound because it is two different elements bonded.
- Nitrogen (N2) is a molecule.
- Water (H2O) is a compound.
- Ozone (O3) is a molecule.
- Sugar (C12H22O11) is a compound.
Compound Examples
Just in case the difference is still a little fuzzy, you can look at a few more examples of compounds.
- salt (NaCl)
- vinegar (C2H4O2)
- ammonia (NH3)
- baking soda (NaHCO3)
- methane (CH4)
Difference Between a Molecule and Compound
Your head is swimming with atoms, compounds, and molecules. Keep everything straight with this simple breakdown of the difference between molecules and compounds.
Molecule | Compound | |
What they are | two or more atoms bonded together | two or more different elements bonded together |
Structure | group of atoms bonded | matter in complete shape (i.e. table salt) |
Relationship | Not all molecules are compounds. | All compounds are molecules. |
Visibility | not visible to humans | visible to humans |
Example | oxygen you breathe (O2) | table salt (NaCl) |
Molecule vs. Compound vs. Element vs. Mixture
Chemists like to throw around a lot of different terminology. Keeping it all straight can be hard. Since you have a clear understanding between molecule and compound, check out how they are different from an element and a mixture by reviewing all their definitions.
- Molecule is a substance with two or more atoms bonded together such as the oxygen humans breathe (O2).
- Elements are pure substances made up of all the same atoms such as gold (Au), hydrogen (H), and oxygen (O). Elements all have the same number of protons in their nuclei and can’t be broken down.
- Compounds are two or more elements bonded together such as table salt (NaCl).
- Mixtures happen when two or more things are combined together but don’t chemically bond, such as salt water or anything you mix in your blender.
Chemistry is full of unique terminology, and now you know how they are different.
Understanding Molecules vs. Compounds
You might have noticed chemistry terms build on one another. A structure with protons, electrons, and neutrons is an atom. Atoms with all the same protons are elements. Atoms that bond are molecules. And finally, different elements that bond are compounds. But without the atom, it would all tumble down.
Now that you’ve explored the difference between molecule and compound, you can look at the difference between an atom and element. You might also find it interesting to explore the difference between an atom and a molecule.