
What Are Ionic Crystals?
Ionic crystals are crystalline structures that grow from ionic bonds and are held together by electrostatic attraction. Ionic bonds are atomic bonds created by the attraction of two differently charged ions. The bond is typically between a metal and a non-metal. Explore several common ionic crystal examples found in nature.
Common Ionic Crystal Examples
The most prevalent ionic crystal example is table salt, or sodium chloride (NaCl). Sodium chloride is created when sodium and chlorine create an ionic bond to become a crystal that tastes great on food. Other examples include:
- potassium fluoride (KF) - the ionic bond of potassium and fluorine
- potassium chloride (KCl) - the bond of potassium and chlorine
- potassium bromide (KBr) - potassium and bromine bonded
- potassium iodide (KI) - the bond of potassium and iodine
- sodium fluoride (NaF) - sodium and fluorine bonded together
- sodium bromide (NaBr) - the ionic bond of sodium and bromine
- sodium iodide (NaI) - a bond of sodium and iodine
- cesium fluoride (CsF) - cesium and fluorine bond
- cesium bromide (CsBr) - a bond created from cesium and bromine
- cesium chloride (CsCl) - the ionic bond of cesium and chlorine
- caesium iodide (CsI) - when caesium and iodine bond
- rubidium fluoride (RbF) - salt created from rubidium and fluorine
- rubidium bromide (RbBr) - an ionic crystal of rubidium and bromine
- rubidium chloride (RbCl) - bond created from rubidium and chlorine
- rubidium iodide (RbI) - rubidium and iodine making an ionic crystal example
- lithium fluoride (LiF) - when lithium and fluorine create a compound
- lithium bromide (LiBr) - the combination of lithium and bromine into a salt
- lithium chloride (LiCl) - an ionic crystal from lithium and chlorine
- lithium iodide (LiI) - the combination of lithium and iodine.
While many of these might not look familiar, you do use them in your everyday life. For example, KCl is used in medicine as a treatment for potassium loss, while sodium fluoride can be found in drinking water and toothpaste.
Understanding Ionic Crystals
As solids, ionic crystals are insulators. As Encyclopedia Britannica states:
“In insulators, nearly all the electrons are bound, and very few electrons are capable of carrying current. A typical metal has one or more conduction electrons in each atomic unit cell, a semiconductor may have only one conduction electron for each thousand unit cells, and an insulator may have one conduction electron per one million or one trillion unit cells.”
However, this isn’t the only key feature of an ionic crystal. They have other unique features as well.
Crystal Lattice
The way that ionic crystals structure themselves when they bond is called a crystal lattice. This is because the ions organize themselves into a regular lattice shape.
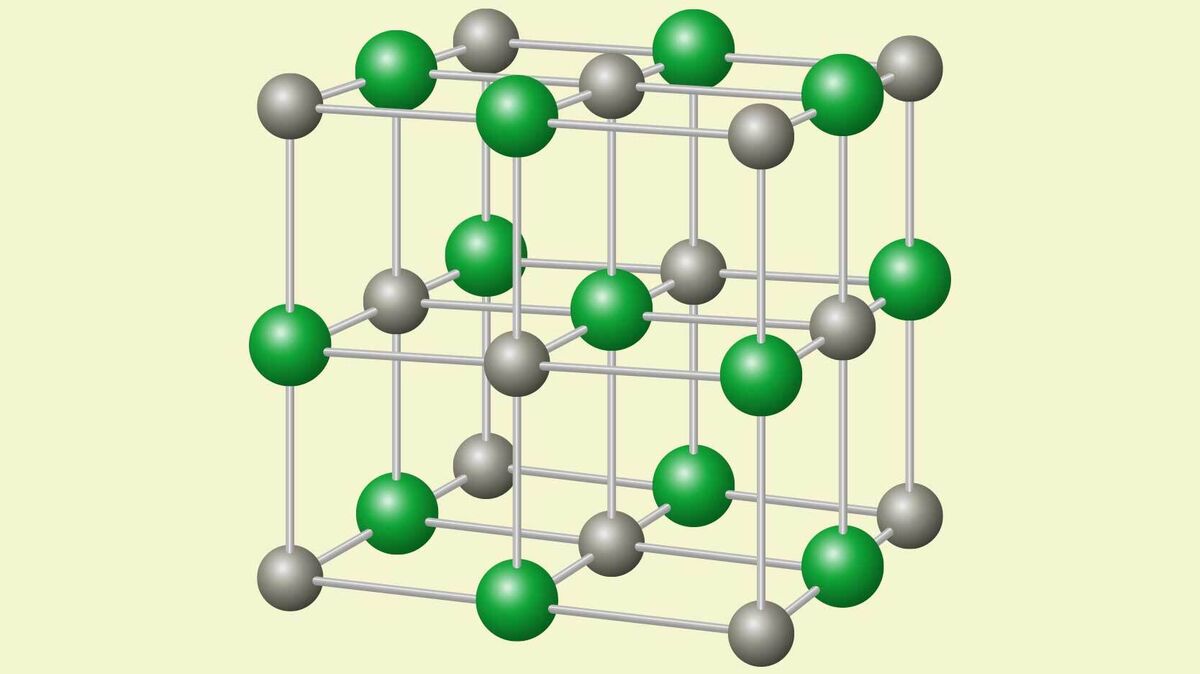
High Melting Point
Ionic crystals melt or boil only at very high temperatures. The enthalpy of these crystals (the amount of fusion and vaporization necessary to melt one mole) is as much as 100 times that of other molecular compounds.
Hard Structures
Ionic crystals are very hard. They are so brittle because if one layer of ions must move past another, the entire arrangement is disturbed.
Seeing Ionic Crystals in Action
Ionic crystals are formed from ions through electrostatic attraction. One of the most well-known ionic crystals is table salt. Learn more about chemistry by exploring examples of chemical bonds.