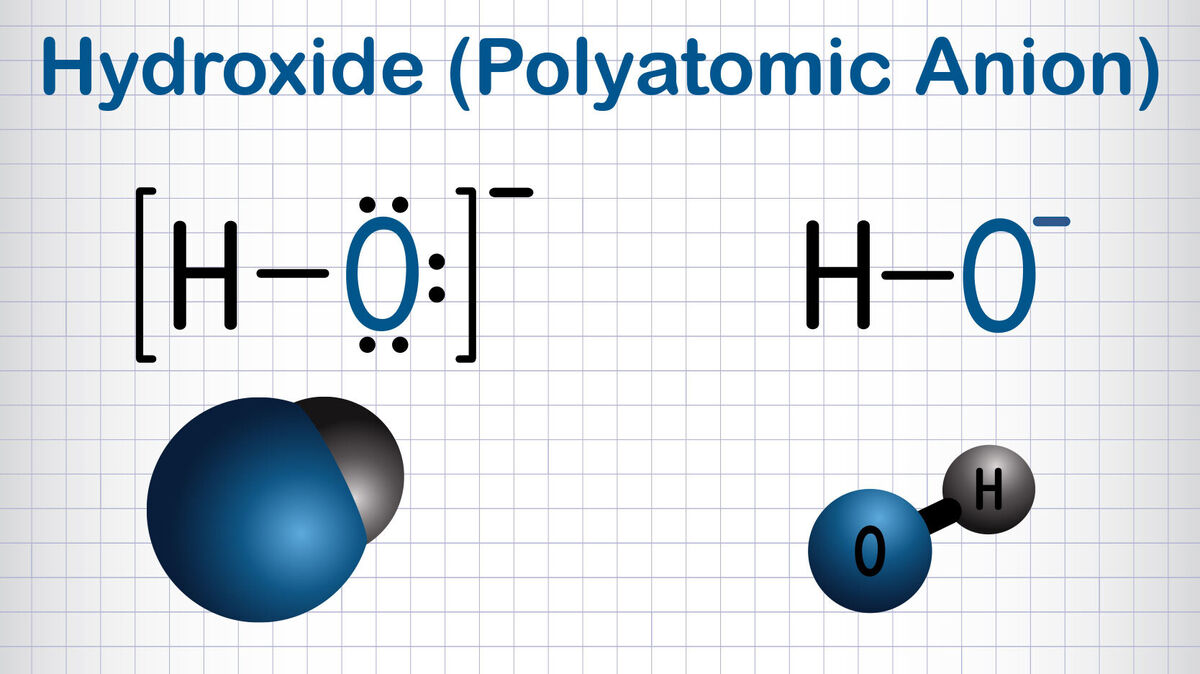
An ion is defined as an atom or group of atoms where the number of electrons is not equal to the number of protons. Electrons have a negative charge, whereas protons have a positive charge. When an atom gains electrons, this results in a negative charge. This type of ion is called an anion. When an atom loses electrons, this results in a positive charge. A positively charged ion is called a cation. Enjoy exploring several examples of ions of both types.
Examples of Positive Ions
Positive ions are typically metals or act like metals. Many common materials contain these ions. Mercury is found in thermometers, for instance, and aluminum is a metal that is found in a surprising amount of things. It's even an ingredient in baking soda and in certain other food products!
The positive charge (more protons versus electrons) for a cation is shown by a number and plus sign after the formula. If there's just a plus sign, it means the charge is plus 1. Review some examples of cations, or positive ions.
- aluminum Al3+
- barium Ba2+
- bismuth Bi3+
- cadmium Cd2+
- calcium Ca2+
- cesium Cs+
- chromium (III) Cr3+
- cobalt Co2+
- copper (I) Cu+
- copper (II) Cu2+
- hydrogen H+
- iron (II) Fe2+
- iron (III) Fe3+
- lead (II) Pb2+
- lithium Li+
- magnesium Mg2+
- mercury (I) Hg22+
- nickel Ni2+
- potassium K+
- rubidium Rb+
- silver Ag+
- sodium Na+
- strontium Sr2+
- tin (II) Sn2+
- zinc Zn2+
Examples of Negative Ions
Just as atoms can lose electrons to become cations, some can gain electrons and become negatively charged anions. Again, you may be familiar with some of these ions. Fluoride is sometimes added to community water supplies. Your dentist may also give you a fluoride treatment.
The negative charge (fewer protons than electrons) for an anion is shown by a number and minus sign after the formula. If there's just a minus sign, it means the charge is minus 1. Review several examples of anions.
- bromide Br-
- chloride Cl-
- fluoride F-
- iodide I-
- nitride N3-
- oxide O2-
- sulfide S2-
Polyatomic Cations and Anions
If an ion consists of two or more atoms, it is called a polyatomic ion. Just like their single-atom counterparts, they too can gain and lose electrons.
Polyatomic Cations
Ions with multiple atoms that lose electrons, and are thus positively charged, are called polyatomic cations.
- Ammonium NH4+
- Hydronium H3O+
Polyatomic Anions
Ions with multiple atoms that gain electrons, and are thus negatively charged, are called polyatomic anions. Per standard notation, the charge is written as superscript text.
- acetate CH3COO- or C2H3O2-
- arsenate AsO43-
- bicarbonate or hydrogen carbonate HCO3-
- borate BO33-
- carbonate CO32-
- chlorate ClO3-
- chlorite ClO2-
- chromate CrO42-
- cyanide CN-
- dichromate Cr2O72-
- dihydrogen phosphate H2PO4- or H2O4P-
- formate CHO2- or HCOO- or CHOO-
- hydrogen sulfate or bisulfate HSO4-
- hydrogen sulfite or bisulfite HSO3-
- hydrogen phosphate HPO42-
- hydroxide OH-
- hypochlorite ClO-
- nitrate NO3-
- nitrite NO2-
- oxalate C2O42-
- perchlorate ClO4-
- permanganate MnO4-
- peroxide O22-
- phosphate PO43-
- phosphite PO33-
- silicate SiO32-
- sulfate SO42-
- sulfite SO32-
- thiocyanate SCN-
- thiosulfate S2O32-
Ionic Compounds
An ionic compound is made up of one or more anions and one or more cations. Explore some examples of ionic compounds.
- aluminum sulfide Al2S3
- beryllium chloride BeCl2
- boron iodide BI3
- calcium nitride Ca3N2
- copper phosphide Cu3P
- iron (II) iodide FeI2
- iron (III) oxide Fe2O3
- lead (II) sulfide PbS
- lead (IV) phosphide Pb3P4
- lithium fluoride LiF
- magnesium chloride MgCl2
- potassium bromide KBr
- sodium fluoride NaF
- sodium nitride Na3N
Fully Charged Reaction
When you study chemistry, you will encounter many examples of ions, as well as the different types of ions and how they interact and relate to each other. Now that you know what is an ion and have reviewed quite a few examples, it's time to learn even more on the topic. Be sure to explore some examples of chemical bonds and examples of chemical properties. Perhaps they'll be the catalyst for positive change in your learning experience!