From the air you breathe to the sugar making those cookies so sweet, molecules are everywhere. Use molecule examples to get a clear picture of what a molecule is and how it differs from an atom, element, or compound.
A Look at Molecules
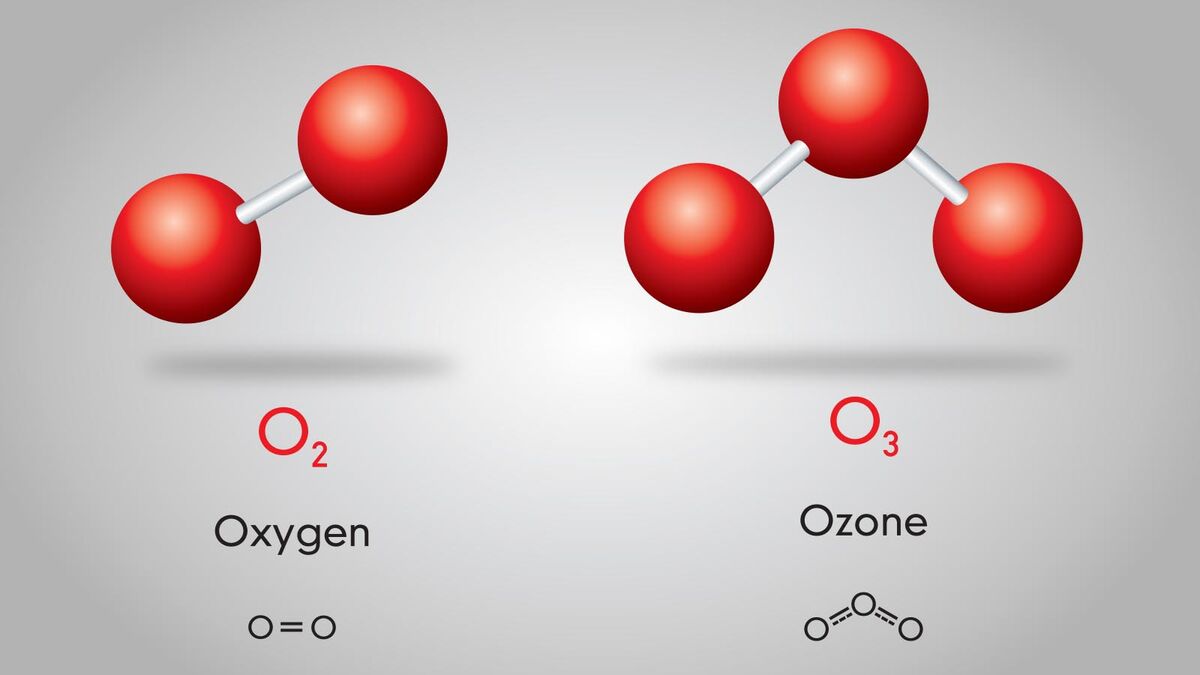
Chemistry can get confusing. Terms like atoms, compounds, elements, and molecules are thrown around all the time. But what exactly is a molecule? In simple terms, a molecule is an atom that forms a chemical bond with another atom. This could be another atom of itself in the case of the oxygen gas humans breathe (O2), or it could be bonding with another atom as in when two hydrogen atoms chemically bond with oxygen to make water (H2O). See this in action through examples.
Molecule Examples
Molecules come in all different shapes and sizes. You might have a small molecule like carbon dioxide (CO2). Then again, a molecule might be huge like hemoglobin (C2952H4664O832N812S8Fe4). Imagine trying to write that chemical formula a few hundred times! While most aren’t that big, explore a few common molecule examples you might know.
- Acetic acid - CH3COOH
- Benitoite - BaTiSi3O9
- Caffeine - C8H10N4O2
- Calcium hydroxide - Ca(OH)2
- Chlorine - Cl2
- Dieldrin - C12H8Cl6O
- Estradiol - C18H24O2
- Fool’s Gold (Iron Sulfide) - FeS2
- Glucose - C6H12O6
- Gypsum - CaSO4
- Hydrogen - H2
- Hydrogen peroxide - H2O2
- Ibuprofen - C13H18O2
- Methane - CH4
- Naproxen - C14H14O3
- Ozone - O3
- Penicillin - C16H18N2O5S1
- Sugar - C12H22O11
- Sulfur - S8
- Sulfuric acid - H2SO4
- Table salt - NaCl
- Testosterone - C19H28O2
- Vitamin B1 - C12H19OS
- Zircon (diamond substitute) - ZrSiO4
While these are just a sampling of common molecules, there are a lot of molecules. Some you know, while others might be a bit more uncommon like ascidiacyclamide benzene solvate (C36H52N8O6S2), which is found in coral reef systems.
Molecule vs. Compound Examples
Now that you have molecules down to a science, it’s time to examine what a compound is. A compound is actually a type of molecule. However, to be a compound, the atoms bonding together need to be different from one another. For example, O2 is a molecule but not a compound. Why? Because it’s bonding with another oxygen atom. On the other hand, NaCl is a compound because it is two different atoms chemically bonded together. Other examples of compounds include:
- Alcohol - C2HO6
- Ammonia - NH3
- Baking soda - NaHCO3
- Butane - C4H10
- Camphor - C8H18
- Citric Acid - C6H8O7
- Ethanol - C2H6O
- Glucose - C6H12O6
- Ethanol - C2H6O
- Methane - CH4
- Nitrogen - N2O
- Octane - C8H18
- Vinegar - C2H4O2
Non-Examples of a Molecule
Having a clear understanding of what makes a molecule and a compound is important. Understanding what is not a molecule is just as important. In addition to molecules, there are atoms. Atoms make up a molecule but they are not a molecule. Find out why.
Atoms
An atom is the smallest particle of matter. It includes protons, electrons, and a nucleus. So, atoms are your starting units for a molecule. For example, a single hydrogen atom (H) is not a molecule; instead, it would be called an atom. However, if you add two hydrogen atoms together (H2), you now have a molecule. Other non-examples include:
- Argon (Ar)
- Calcium (Ca)
- Iron (Fe)
- Neon (Ne)
The Wacky World of Molecules
When it comes to chemistry, things can get confusing fast. Just the fact that atoms are made up of molecules and compounds are a type of molecule is enough to make your head spin. Add into that the finer details of elements, and knowing what is a molecule and what isn’t can get very confusing. Thankfully, molecule examples work to clear everything up. Ready to learn more about compounds? Dive into examples of organic compounds and hydrogen bond examples. Chemistry is fun!