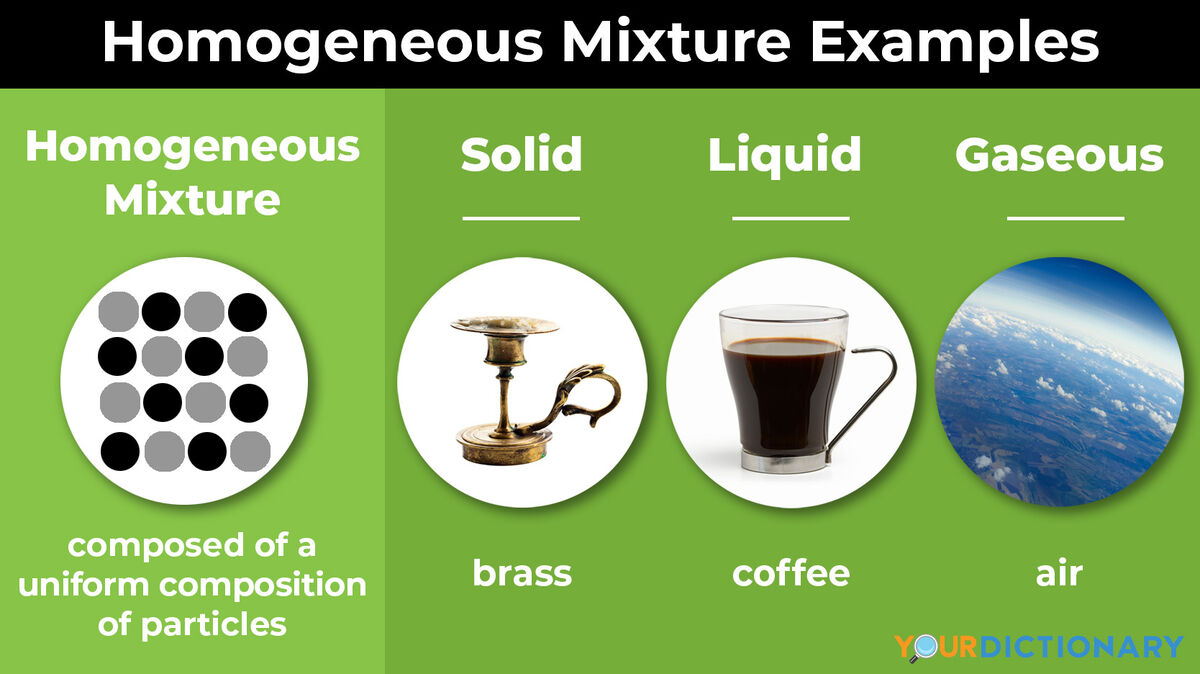
A chemical mixture combines two substances that maintain their own properties when combined. Heterogeneous mixtures are made up of a non-uniform composition, while homogeneous mixtures are made up of a uniform composition. For example, water and sand is a heterogeneous mixture — you can easily separate the sand from the water. But orange juice is homogenous — it would be difficult, if not impossible, to separate the orange particles from the water. Keep reading for more examples of solid, liquid and gaseous homogenous mixtures that you see every day.
Solid Homogeneous Mixture Examples
Homogeneous mixtures are also known as solutions. When you think of a solution, you probably think of a liquid. However, many solids are also considered homogenous mixtures. There is a wide variety of solid homogeneous mixtures, from naturally occurring materials like stone to synthetic plastics.
- Bitumen - the solid form of petroleum and source of gasoline, diesel and other fossil fuels; bitumen is a homogeneous mixture of complex hydrocarbon chemicals
- Cement - a solid homogeneous mixture of calcium compounds; mixed with sand, gravel and water, it becomes the heterogeneous mixture concrete, one of the most important building materials in the world.
- Bronze - a mixture of copper and tin; bronze is a type of alloy, which is a metal created by two or more metallic elements
- Steel - an alloy made from iron and carbon; both steel and stainless steels, which includes chromium, are homogeneous mixtures
- Brass - a metal made with copper and zinc; brass is also an alloy
- Thermoplastics - a type of manufactured plastic that includes polyethylene and polyvinyl chloride; thermoplastics include polymers that heat and cool uniformly into solid plastic substances
You may be wondering if certain types of stone, such as granite, are homogeneous. Rocks are made out of different minerals, crystals and substances, making them heterogeneous. However, the minerals that compose rocks are often homogeneous themselves.
Liquid Homogeneous Mixture Examples
Many of the liquids you encounter every day are examples of homogeneous mixtures. These liquids include the beverages you drink, your bodily fluids and household cleaning materials.
- Blood plasma - the colorless fluid that holds your blood cells in suspension; it makes up a little more than half of the volume of human blood; Blood itself appears homogeneous, but the cells and the plasma can be easily separated, making it heterogeneous.
- Wine - a homogeneous mixture (like all liquors); the science of making wine and liquor is based on employing ethanol and/or water as a solvent on various substances — charred oak for bourbon whiskey, for example, or juniper in gin — to create unique flavors
- Water - another example of homogeneous mixture; all but the purest water contains dissolved minerals and gases; These are dissolved throughout the water, so the mixture presents in the same phase and is homogeneous.
- Liquid laundry detergent - a homogeneous mixture of various soaps and chemicals for washing clothes; you cannot easily separate the soap from the water in concentrated laundry detergent
- Coffee - a homogeneous mixture of water and filtered coffee grounds; while there may be some solids left over from the brewing process, you cannot easily separate the coffee from the water, making it mainly homogeneous
- Saline solution - sodium chloride (salt) and water mixed together; saline has the same concentration of salt (0.9%) as blood and tears, making it ideal for medical purposes
Some people argue that homogenized milk — milk that has been treated by a machine to ensure that fat molecules are consistent throughout the liquid — is homogenous. While the substances (fat and water) will not separate in homogenized milk, it is technically a colloid. The fat is suspended rather than dissolved; therefore, milk is a heterogeneous liquid suspension of fats in water.
Gaseous Homogeneous Mixture Examples
Many of the most common gaseous substances people encounter are homogeneous mixtures. Take a breath — you're breathing in a gaseous homogeneous mixture!
- Air - a homogeneous mixture of oxygen, nitrogen, argon, and carbon dioxide, along with other elements in smaller amounts; because each layer of the Earth's atmosphere has a different density, each layer of air is its own homogeneous mixture
- Natural gas - a gaseous homogeneous mixture of methane and other hydrocarbons used as a fuel; you can't separate out the parts of natural gas
- Nitrous oxide - one of many gaseous homogeneous mixtures used for anesthesia; as anesthesia, nitrous oxide is used in a 50/50 solution with oxygen; In fact, doctors colloquially refer to nitrous oxide as "gas and air!"
When the air around you contains droplets of moisture (such as in fog or mist), it's considered heterogeneous. The same goes for when you sneeze or spray perfume into the air.
Single Phase vs. Multi-Phase
Another key component of homogenous mixtures vs. heterogeneous mixtures is what phase each substance is in. For example:
- Homogeneous mixtures include single-phase substances (the same state of matter), such as coffee with creamer (both liquid) or sterling silver (made with silver and copper).
- Heterogeneous mixtures include multi-phase substances (different states of matter), such as sand and water (solid and liquid) or carbonated drinks (gas and liquid).
It's possible for a mixture to start out as heterogeneous and then become homogeneous as one substance dissolves. An example would be adding sugar to water; the sugar crystals are solids, but when they dissolve, the sugar is in liquid form.
Understanding Homogeneous Mixtures: The Matter of Mixtures
Understanding homogeneous and heterogeneous mixtures is vital to building your knowledge of chemistry. Examples of homogeneous mixtures help reveal the remarkable scientific secrets that inform even the simplest parts of life. Next, take a look at some examples of physical properties of matter. You can also check out these examples of chemical changes that occur in your everyday life.