Electrolytes are chemical compounds in the form of minerals that serve several interesting purposes. Electrolytes are necessary for the human body to function, but that is not all they do. Chemical compounds that are examples of electrolytes are also necessary for batteries to function; some are even included in rocket fuel. How is that for diverse functionality? Learn what electrolytes are and review some examples.
What Are Electrolytes?
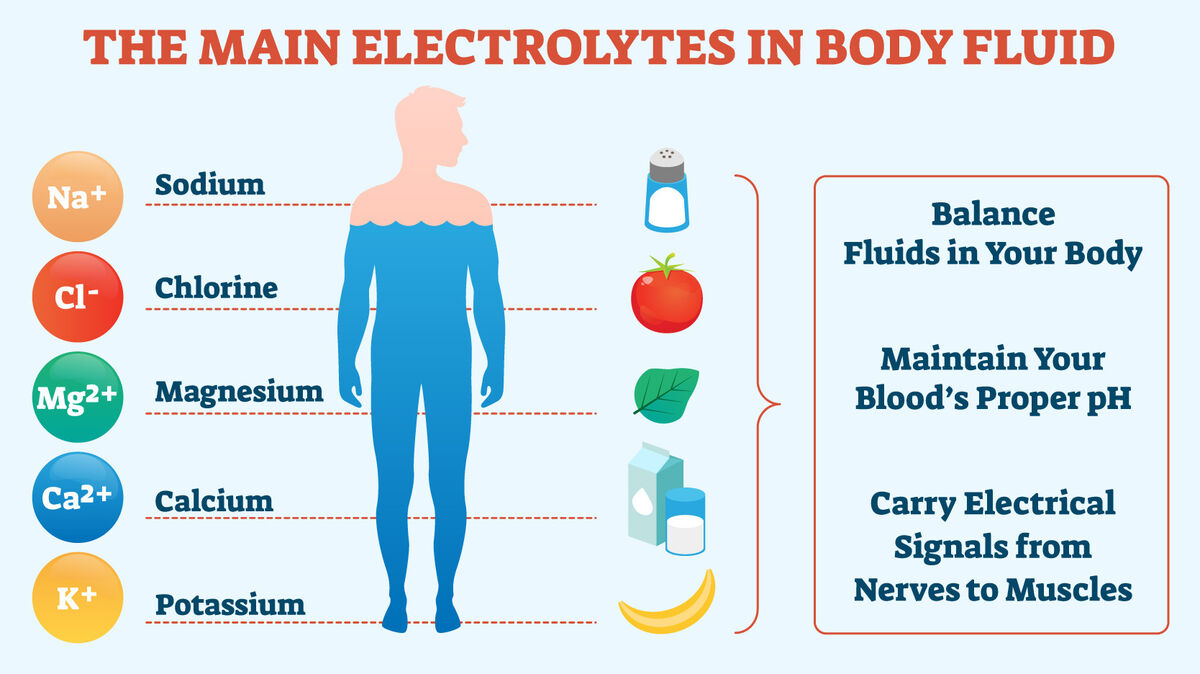
Electrolytes are examples of minerals. They are distinguished from other minerals in that they carry an electrical charge when dissolved in water. Humans and animals alike need electrolytes for their bodies to function. Electrolytes can be found in blood, urine, other bodily fluids, and tissues.
Importance of Electrolytes to the Body
Electrolytes fulfill several important purposes for people and animals.
- regulate the body's pH level, which ensures a proper balance between acid and base in the body
- balance the quantity of water that is present in the body
- assist with the flow of fluid between the body's cells
- move substances into and out of cells, thus ensuring that nutrients go in and waste goes out
- enable key systems of the body, including the nervous, muscular, cardiovascular, and central nervous systems, to function properly
Dangers of Electrolyte Imbalance
If the levels of electrolytes in an individual's body get too high or too low, this creates a problem. If someone's electrolyte level becomes too low, that could lead to dehydration. Someone who has an electrolyte level that is too high can become overhydrated. Both situations can be dangerous; an electrolyte imbalance can keep the body from functioning as it should.
An electrolyte imbalance can be caused by anything that disrupts the balance of water in the body. For example, sweating due to heavy exertion can cause electrolyte levels to become low. This is why athletes and those who exercise vigorously can benefit from electrolyte-containing drinks, such as Gatorade and Powerade. Other common causes of electrolyte imbalance include diarrhea, vomiting, kidney function issues, and medications that cause one to retain or lose water.
Examples of Electrolytes in the Body
The body needs a number of electrolytes. Eating foods or drinking beverages that have these substances is the primary way that people or animals take in the electrolytes they need.
- calcium
- potassium
- chlorine
- magnesium
- sodium
- phosphate
List of Chemicals That Are Electrolytes
The electrolytes that tend to be present in the bodies of humans and animals are not the only ones that exist. Many chemical compounds are electrolytes and can be used for a variety of purposes. Some are food additives while others are substances used in industrial applications. Many should not be consumed or even touched directly.
- sodium chloride - Also known by the formula NaCl, sodium chloride is a compound with equal parts sodium and chloride. It is more commonly known as salt or table salt. It is the major ingredient in edible salt used to make food taste better. It is present in the ocean and is the main reason ocean water tastes salty.
- nitric acid - This strong mineral acid, known by the formula HNO3, is a corrosive acid commonly used in some types of fertilizer. It has also been used as one of the ingredients in certain types of liquid rocket fuel. It's also a chemical used in woodworking to make wood look like it has been aged.
- chloric acid - HClO3 is the formula for chloric acid, which is a very dangerous oxidizing agent. Chloric acid can be produced by a chemical reaction.
- hydrochloric acid - This strong acid is widely used in the chemical industry, but is also an ingredient in gelatin, leather and household cleaning supplies.
- calcium chloride - Although calcium chloride is a "salt" by definition, it is different from the table salt described above as sodium chloride. Made of calcium and chloride, this is one of the types of salt used to control ice on sidewalks and roadways. The compound is often produced from limestone.
- potassium nitrate - Potassium nitrate, commonly known by the formula KNO3, is used in a wide variety of substances. It can be used as a food additive but is also an ingredient in some types of rocket fuels and fireworks. Potassium nitrate was once known as saltpeter, which was an ingredient in gunpowder for many years.
- sodium hydroxide - With many uses throughout history, sodium hydroxide — also known as lye — is an important ingredient in many detergents, soaps and drain cleaners. It is highly dangerous because of its ability to decompose lipids and proteins in the skin, causing burns when not handled properly.
- sulfuric acid - Once known as oil of vitriol, this highly corrosive strong acid (known by the molecular formula H2SO4) can corrode metals, organic compounds, living tissues, and even stone. It is used as an electrolyte in certain types of car batteries to get the electricity to flow. It can cause very severe chemical burns on skin.
- sodium acetate - Sodium acetate is often used to seal concrete to protect it against bad weather. It is also an ingredient in certain types of foods, such as salt and vinegar potato chips, because of its salty and tangy flavor when mixed with other seasonings.
- magnesium hydroxide - Because of its milk-like appearance, this electrolyte was long known by the name milk of magnesia. It is a main component of many types of laxatives, antacids, underarm deodorants, and antiperspirants. It can also be applied to the scalp as a form of seborrhea and dandruff control.
Learn About Substances the Body Needs
While you're learning about electrolytes, it's a good time to better educate yourself about other substances the body needs, including protein and complex carbohydrates. Explore some examples of protein in biology and diet. Then, find out which foods contain complex carbohydrates.