Chemical and physical changes happen all the time in our everyday lives. But how can you tell if an item or substance has undergone a chemical change or a physical change, and what’s the difference? Keep reading to tell the difference between these changes with explanations and examples of each.
Chemical Change vs. Physical Change
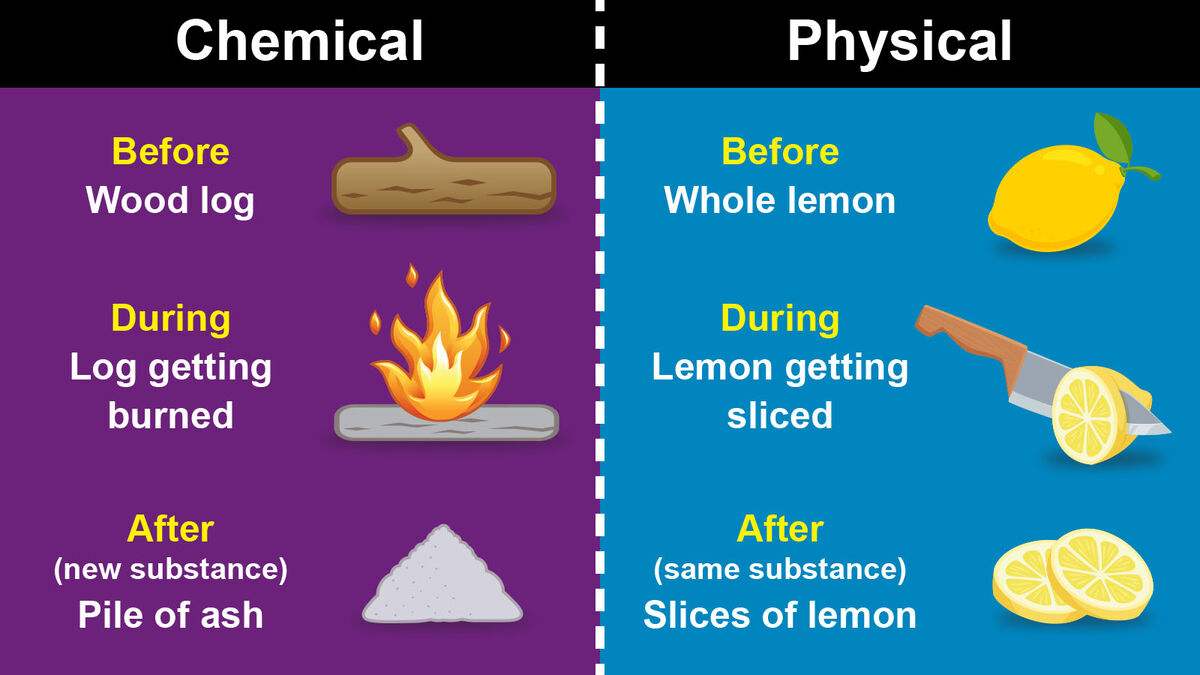
Telling the difference between a chemical change and a physical change seems trickier than it is. The main difference between a chemical change and a physical change is what happens to a substance’s composition. Here are the basic definitions of chemical and physical changes:
- chemical change – a process in which chemical bonds are broken or created to make a new substance
- physical change – a process in which a substance changes its state of matter, but chemical bonds stay intact
When matter undergoes a chemical change, it can’t return to its original state without additional reactions. But when it undergoes a physical change, it only needs to return to its original state of matter. The molecules of the substance haven’t been changed at all.
Examples of Chemical Changes
Chemical changes occur all around you in everyday life. Whenever a chemical reaction occurs, the chemical properties of the original substance change to create an entirely new substance. Some examples of chemical changes include:
- Iron rusting
- Burning firewood
- Grilling meat
- Organic matter decomposing
- Fruit ripening
- Food being digested
- Frying an egg
- Baking cookies
- Photosynthesis
- Tarnished silver
- Food spoiling
- Teeth decaying
- Wine fermenting
- Firing clay in a kiln
- Respiration (breathing)
- Fireworks exploding
Most of these changes cannot be undone after a chemical reaction. While you can use silver polish to remove tarnish with another chemical reaction, for example, you can’t unfry an egg or reverse the decomposition process of a dead leaf. Determining whether a change can be undone is a key factor in deciding whether an item’s change is chemical or physical.
Examples of Physical Changes
Physical changes involve an object or substance changing shape or state of matter. Even though it now has a different physical property, it’s still the same object or substance – its molecules are still the same. Here are some examples of physical changes you might see:
- Freezing water to make ice
- Melting ice to make water
- Heating water to make steam
- Reshaping soft clay to make another shape
- Crumpling a piece of paper
- Bending a paper clip
- Creating mud with dirt and water
- Chopping vegetables
- Breaking a pencil in half
- Dissolving sugar into water
- Eroding rocks on the coastline
- Breaking a glass window
- Tempering steel
- Cutting fabric
While all of these processes are physical changes, some are easier to reverse than others. It’s much easier to melt an ice cube than to put a carrot back together, for instance. But even though the pieces of a carrot are smaller than a whole carrot, the molecules of each piece are unchanged – it’s still a carrot.
Both Chemical and Physical Changes?
Think about a process like chewing your food. The food that is crushed by your teeth has the same molecular structure as it did before you took your first bite, making it a physical change. However, as soon as your saliva hits these pieces of food, enzymes begin breaking it down, which is a chemical change.
You’ll find that many everyday processes, especially biological functions, include both physical changes and chemical changes. The key is remembering when one process stops and the next begins.
The Chemistry of Everyday Life
Although physical changes are sometimes easier to see, chemical changes are more dramatic at the molecular level. They create new substances as the result of chemical reactions. Read another resource for more examples of simple chemical reactions in everyday life.