What’s the difference between an atom and molecule? Molecules are made of atoms. While that might be the biggest difference between molecules and atoms, it isn’t the only difference. Dive deep into atoms and molecules to find what makes them each unique.
What’s an Atom?
Something always must come first right? It might be an age-old question of which came first, such as the chicken or the egg. But when it comes to an atom vs. molecule, you know the atom came first. Now the only question is why? To know the answer to that question, you need to look at the structure of an atom.
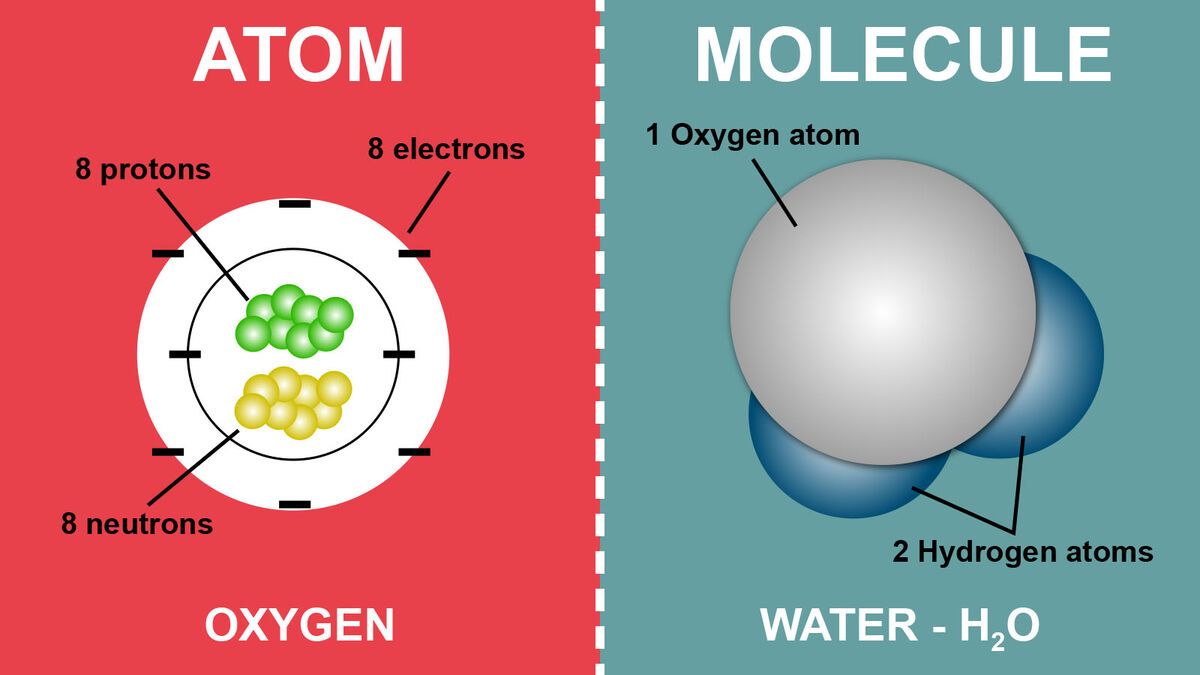
An atom is the smallest unit of matter. It’s composed of different particles called electrons, protons, and neutrons. While there are other fun subatomic particle names thrown in there, those are the three most important ones.
- Neutrons, like their name, are neutral in charge, meaning they don’t have one.
- Protons have a positive charge.
- Electrons hold a negative charge.
Protons and neutrons are found in the atomic nuclei while electrons orbit the nucleus.
Now, let’s explore the structure of a molecule.
What’s a Molecule
Here is where it gets interesting. A molecule is made up of atoms bonded together. So, while an atom is its own separate entity, a molecule is what you get when those atoms bond together. These might be the same elements, such as two oxygen atoms bonded together (O2), or it might be different atoms bonded together like water (H2O). If three oxygen atoms bond together, you get the molecule ozone (O3).
Molecules can get complex, too. For example, the molecule acetone is made of carbon, hydrogen, and oxygen. Therefore, it has the chemical formula (CH3)2O.
Now that you understand the structural difference, let’s see how an atom becomes a molecule.
From Atoms to Molecules
Why do atoms form molecules? It all comes down to those positively and negatively charged subatomic particles in an atom. If a proton or electron is lost, an atom can become negatively or positively charged. When this happens, an atom becomes unstable. Well, atoms like balance. Therefore, when an atom becomes negatively or positively charged, they can attract one another to become balanced again. For example, they might share an electron to form a chemical bond. This can be a single bond such as in the case of ammonia (NH3). However, molecules can also form double and triple bonds.
Breaking Down Atoms and Molecules
As you’ve probably already guessed, atoms can’t be broken down. Atoms are in their simplest form. However, since molecules are created from atoms, molecules can be broken down into their individual atoms using chemical reactions to break their bonds. For example, water is made up of hydrogen and oxygen. Therefore, H2O can be split into its individual atoms using electricity through a process called electrolysis.
Difference Between an Atom and Molecule
Atoms and molecules are easy to confuse. However, keep things clear with this table.
Atom | Molecule | |
Definition | Single entity | Made of two or more atoms |
Structure | Made of protons, neutrons, electrons | Made of multiple atoms bonded together |
Stability | Not stable | Stable |
Chemical Reactions | Can’t be separated by chemical reactions | Can be separated by chemical reactions |
Examples | Oxygen (O) | Water (H2O) |
Hydrogen (H) | Sodium chloride (NaCl) | |
Argon (Ar) | Nitrogen (N2) | |
Plutonium (Pu) | Carbon dioxide (CO2) | |
Iron (Fe) | Nitrous oxide (N2O) | |
Calcium (Ca) | Ozone (O3) |
Understanding Atoms and Molecules
Chemistry can be confusing. Especially when you start to get into the different chemical terms. When it comes to the difference between atoms and molecules, just remember molecules are made of atoms. Since you did so well at understanding molecules vs. atoms, you can look at the difference between molecules and compounds. If you are looking for even more chemical excitement, look at atoms and elements. It’s about to get atomic!