Acids and bases are everywhere. However, you are most likely to hear those terms used in chemistry. The difference between acids and bases has to do with how they ionize in water. Keep things crystal clear by breaking down the properties of acids vs. bases. See each different substance through real-world examples.
Difference Between Acids and Bases
Acids and bases play important roles in chemistry, but you can also find them around the house. Therefore, knowing the difference between the two is important for safety.
While there are several key differences in chemical properties between acids and bases, the main one is their pH level. Acids have a pH level lower than 7.0 while bases have a pH level higher than 7.0. Discover why acids and bases have a different pH level along with other important properties by looking at each substance.
What Are Acids and Bases?
How you define an acid or a base depends on three different theories in chemistry: Arrhenius Theory, Bronsted-Lowry Theory, and Lewis Theory.
Acid | Base | |
Elevate the H+ in water | Elevate the H- in water | |
Proton donors | Proton acceptors | |
Accept pairs of electrons | Donate pairs of electrons |
Acid vs. Base pH
However, for those without a science degree, it’s best to remember the pH (power or potential for hydrogen). You can test the pH of something with a pH test strip (litmus paper). Acids turn blue litmus paper red, while bases turn red litmus paper blue. This is because acids have a low pH, while bases have a high pH. You might not realize this, but pH strips are used to test the water in your pool. See? Chemistry doesn’t just happen in a lab.
Quick Breakdown of Properties of Acids and Bases
In addition to defining acids and bases, it’s important to look at their different properties.
Term | Acids | Bases |
Corrosive | yes | yes |
Taste | sour | bitter |
pH | less than 7.0 | more than 7.0 |
Hydrogen ions in water | h+ | h- |
Litmus paper | blue litmus paper turns red | red litmus paper turns blue |
Examples of Acids and Bases
Ready for a few acid and base examples? You’ll be surprised to hear some of these are things you have in your house.
When you think of acids, you might think of solutions that can burn your flesh. However, there are all kinds of acids.
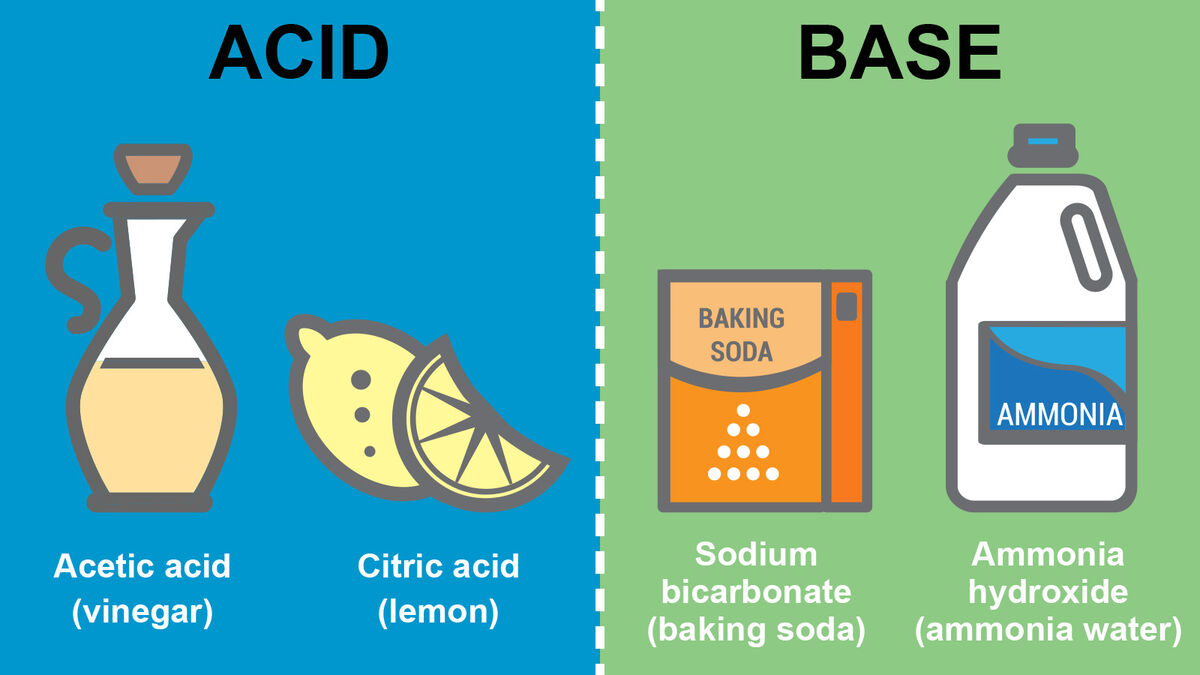
- Citric acid (oranges and lemons)
- Acetic acid (vinegar)
- Hydrochloric acid (stomach acid)
- Carbonic acids (soft drinks)
- Nitric acid
Looking for a common household base? Think baking soda. You might find these other bases as well.
- Ammonia hydroxide (ammonia water)
- Magnesium hydroxide (milk of magnesia)
- Sodium borate (borax)
- Calcium hydroxide (limewater)
- Seawater
Safety With Acids vs. Bases
It’s important to know the difference between acids and bases in terms of safety. Since both strong and weak acids and bases can be corrosive and severely burn skin and eyes, it’s always important to proceed with caution when handling either. Just be aware that:
- Strong acids have a pH of about 1 or less depending on concentration. They can be extremely dangerous and reactive such as the sulfuric acid used in battery acid.
- Strong bases are those with a pH of about 13 or so depending on the concentration. Strong bases include bleach.
Additionally, it’s important to know the difference between acids and bases because mixing the two together can cause a reaction. While mixing vinegar and baking soda can create a great cleaning agent, mixing strong acids and bases can create toxic fumes or even explosions.
Acids and Bases in Chemistry
It’s important to know the difference between acids and bases. This is true in chemistry and even in your own home. Don’t let your science knowledge stop at acids and bases. Keep this learning going through looking at physical and chemical weathering. Learning is fun!