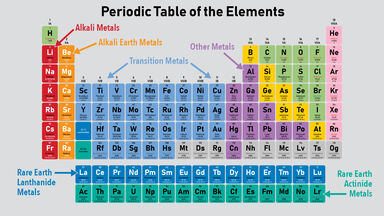
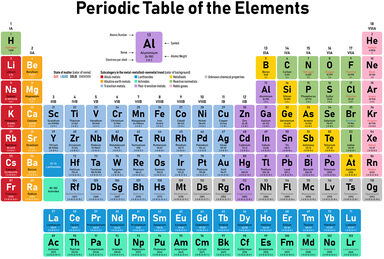
The citrates are a numerous class of salts, the most soluble of which are those of the alkaline metals; the citrates of the alkaline earth metals are insoluble.
When heated with the alkali and alkaline earth metals it yields silicon and the corresponding metallic chlorides.
It is found in the form of oxide (silica), either anhydrous or hydrated as quartz, flint, sand, chalcedony, tridymite, opal, &c., but occurs chiefly in the form of silicates of aluminium, magnesium, iron, and the alkali and alkaline earth metals, forming the chief constituent of various clays, soils and rocks.
The alkali and alkaline earth cyanides are soluble in water and in alcohol, and their aqueous solution, owing to hydrolytic dissociation, possesses an alkaline character.
All the metallic chlorides, with the exception of those of the alkali and alkaline earth metals, are reduced either to the metallic condition or to that of a lower chloride on heating in a current of hydrogen; most are decomposed by concentrated sulphuric acid.