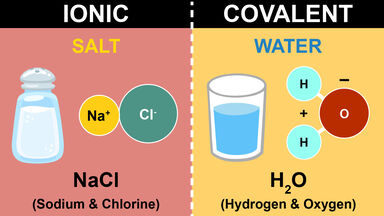
Thus the equation Cl 2 -1-2KI, Aq=2KC1, Aq+12+52400 cal., or (C12) +2KI, Aq =2KC1, Aq+[12]-I-52400 cal., would express that when gaseous chlorine acts on a solution of potassium iodide, with separation of solid iodine, 52400 calories are evolved.
A basic chloride, Pb(OH)Cl, was introduced in 1849 by Pattinson as a substitute for white lead.
A similar expression can be found for Q'P - Q"A; and thus, if Q' A =v, Q' AO = where v =a cos (0", we get - - -AQ' = a sin w (sin 4 -sink") - - 8a sin 4 w(sin cktan 4 + sin 'tan cl)').
This last circuit has a natural frequency of its own which is numerically measured by I/27r-!(CL), where C is the capacity of the condenser and L is the inductance of the circuit.
All these are strikingly alike in appearance and general characters, differing essentially only in chemical composition, and it would seem better to reserve the name cerargyrite for the whole group, using the names chlorargyrite (AgC1), embolite (Ag(Cl, Bl)), bromargyrite (AgBr) and iodembolite (Ag(C1, Br, I)) for the different isomorphous members of the group. They are cubic in crystallization, with the cube and the octahedron as prominent forms, but crystals are small and usually indistinct; there is no cleavage.